药物基本信息
通用名:利妥昔单抗
临床用途:用于复发或耐药的滤泡性中央型淋巴瘤(国际工作分类B、C 和D 亚型的B 细胞非霍奇金淋巴瘤)的治疗。先前未经治疗的CD20 阳性III-IV 期滤泡性非霍奇金淋巴瘤,患者应与标准CVP 化疗(环磷酰胺、长春新碱和强的松)8 个周期联合治疗。CD20 阳性弥漫大B 细胞性非霍奇金淋巴瘤(DLBCL)应与标准CHOP 化疗(环磷酰胺、阿霉素、长春新碱、强的松)8 个周期联合治疗。
市场需求
利妥昔单抗由罗氏生产,2000年即批准进入国内市场,从2010年至今,年平均增长率均在10%以上,2016年全国销售额高达40亿元,是全球TOP10药物中在国内市场表现最好的一款药物。
The basic information of drug
common name: Rituximab
Clinical use: treatment of relapsing or resistant follicular central type lymphoma (B, C and D subtypes of B cells in non - Hodgkin 's lymphoma classified by international work). The previously untreated CD20-positive stage III-IV follicular non-Hodgkin's lymphoma should be treated combining with standard CVP chemotherapy (cyclophosphamide, vincristine and prednisone) for 8 cycles. CD20-positive diffuse large B-cell non-Hodgkin's lymphoma (DLBCL) should be treated combining with standard CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, prednisone) for 8 cycles.
Market demand
Rituximab is produced by Roche, and approved in 2000 to enter the domestic market. From 2010 to date, the average annual growth rate is more than 10%. 2016 national sales are up to 40 billion yuan. It is the best one drug of the global TOP10 drugs in the domestic market.
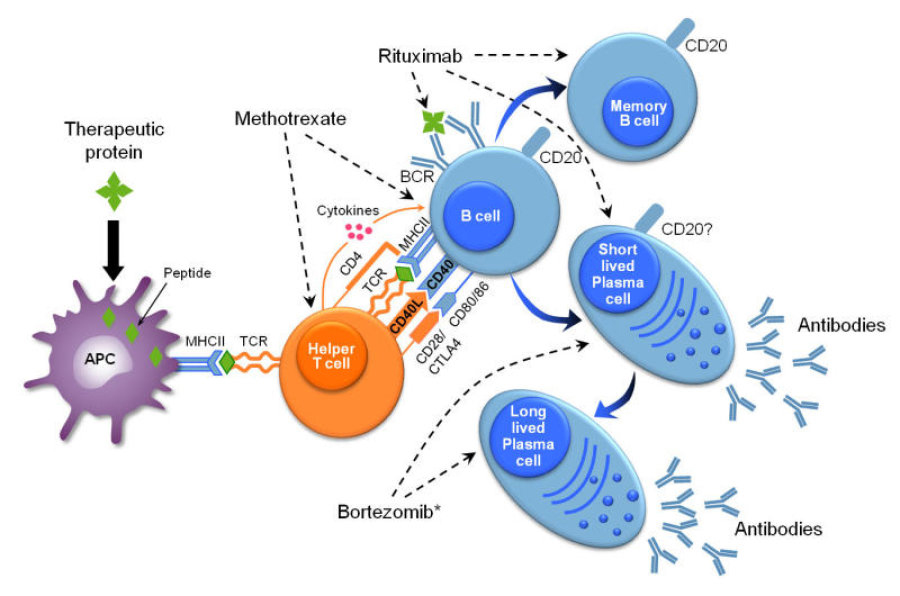 (
( 利妥昔单抗作用机制,图片来源生物谷)





